Healthcare organizations operate in one of the most heavily regulated environments in the world. From ensuring patient safety to meeting strict production standards, businesses must strike a balance between following regulatory rules and building systems that guarantee consistent quality.
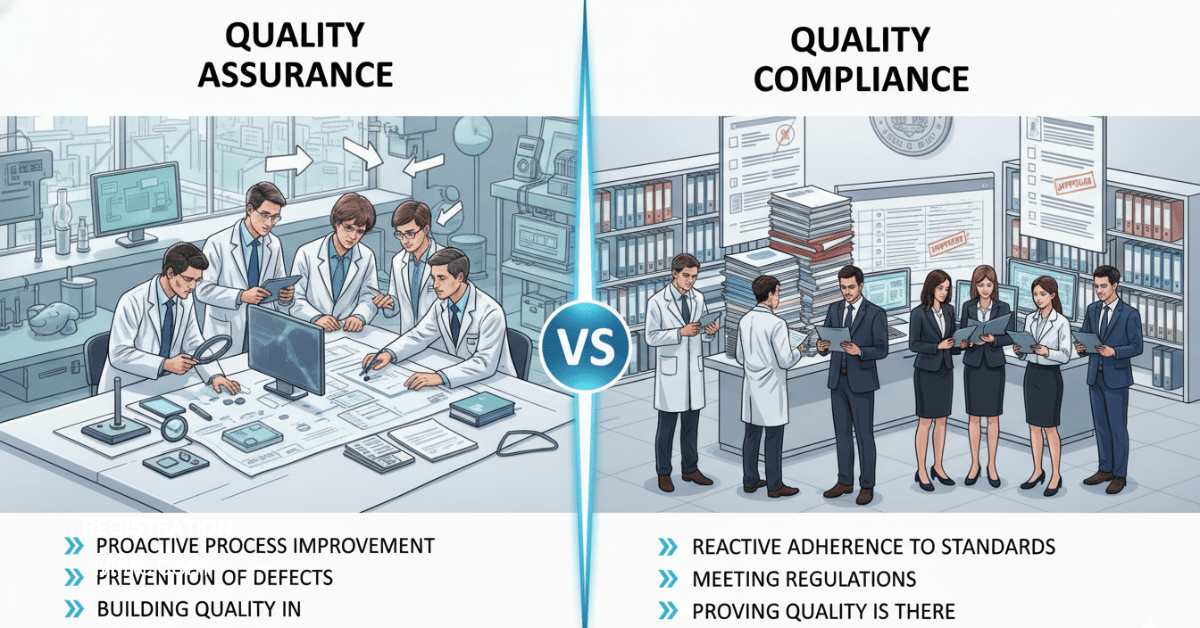
This is where the concepts of compliance and quality assurance come into play. While compliance is about meeting the minimum requirements set by regulators, quality assurance goes deeper by embedding practices that improve reliability and safety.
Understanding how the two differ and why both are necessary helps companies reduce risks, maintain credibility, and deliver products and services that patients and consumers can trust.
Compliance refers to following the external rules, regulations, and laws that govern the healthcare industry. It provides the legal foundation for operations and ensures organizations meet the expectations of regulators and government agencies.
Core aspects of compliance include:
Compliance is not optional. A company that fails to meet these requirements risks facing penalties, license suspension, or even permanent closure.
Quality assurance (QA) is the system of internal processes that ensures products and services meet both industry standards and consumer expectations. Unlike compliance, which is enforced from the outside, QA comes from within the organization. It is designed to reduce errors, prevent risks, and maintain consistency across every stage of production or service delivery.
Key features of QA include:
Quality assurance is about building trust with patients, regulators, and consumers by making sure that what leaves the facility is safe, effective, and reliable every single time.
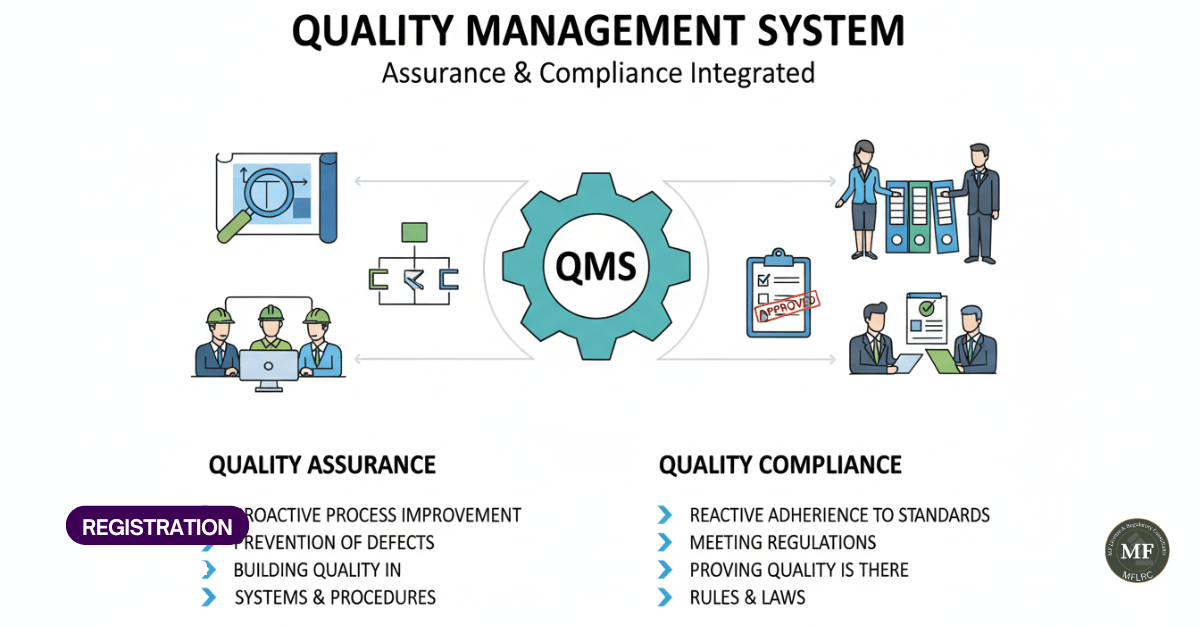
Although both terms often overlap, they have distinct roles. Here are the main differences:
Aspect | Compliance | Quality Assurance |
Focus | Meeting external rules and regulations | Building internal systems and culture |
Purpose | Legal operation and risk avoidance | Consistency, reliability, and patient trust |
Approach | Checklist-driven | Process-driven and preventive |
Impact | Protects from legal or financial penalties | Improves outcomes and reputation |
Scope | Ensures today’s rules are met | Focuses on long-term improvements |
Compliance is the foundation, but quality assurance ensures the structure stands strong in the long run.
Many organizations assume compliance is enough, but without QA, they leave themselves vulnerable to quality failures. On the other hand, focusing only on QA without compliance means they risk losing their license.
Benefits of combining QA and compliance include:
The most successful healthcare companies treat compliance as the baseline and QA as the standard that ensures ongoing excellence.
Balancing these two requirements is not always simple. Healthcare organizations often face:
Overcoming these challenges requires strong leadership, ongoing training, and often external guidance.
To integrate compliance and QA effectively, organizations should consider the following practices:
To see how the two concepts work together, consider these real-world industry applications:
In every sector, compliance gets a company into the market, but QA keeps it there.
MFLRC helps businesses integrate quality assurance and compliance seamlessly across their operations. Our expertise covers industries including pharmaceuticals, cannabis, cosmetics, food, tobacco, and natural health products.
Our support includes:
With our guidance, organizations can meet strict industry standards while building internal systems that ensure consistent safety and reliability.
Compliance and quality assurance may share common ground, but they are not the same. Compliance ensures healthcare businesses meet the external standards required to operate legally. Quality assurance builds the internal systems that consistently deliver safe, effective, and trusted products. When combined, they protect patients, reduce risks, and create sustainable growth opportunities.
Healthcare organizations that view compliance as the foundation and QA as the framework achieve more than just legal approval. They create a reputation for reliability and safety that lasts well into the future. With expert support from MFLRC, businesses can confidently manage both sides of this critical balance.
| Disclaimer |
| The above blog post is provided for informational purposes only and has not been tailored to your specific circumstances. This blog post does not constitute legal advice or other professional advice and may not be relied upon as such. |

MFLRC is a one-stop shop for all of your Licensing, quality assurance and compliance needs. Our team has years of experience in the cannabis industry and are experts in all facets. We offer a variety of services that will save you time and money. Let us take the burden off your shoulders so you can focus on what’s important – growing your business.
Contact us Now!
Mussarat Fatima, President, and owner of MF Cannabis License and Regulatory Consultants has more than twenty years of experience in Quality Assurance, Quality Control, and Regulatory Affairs within the pharmaceutical, Food and Cannabis industries. She has a Master’s Degree in Food Sciences and Biochemistry; in addition to this, she also has a diploma in pharmaceutical Quality Assurance, Regulatory Affairs, and Quality Control. Also, she has completed several certifications specifically in Cannabis Quality Assurance, Regulatory Affairs, and Facility management from recognized institutes in Canada.

Written By: Mussarat Fatima
President at MF License & Regulatory Consultants
Website: https://mflrc.com/
Contact: info@mflrc.com