Recalls are intended to safeguard public health by informing consumers (when necessary) of the presence on the market of a potentially dangerous cannabis product and facilitating the efficient and prompt identification and removal of unsafe items from the distribution chain, ensuring that potentially dangerous products are destroyed or declared safe.

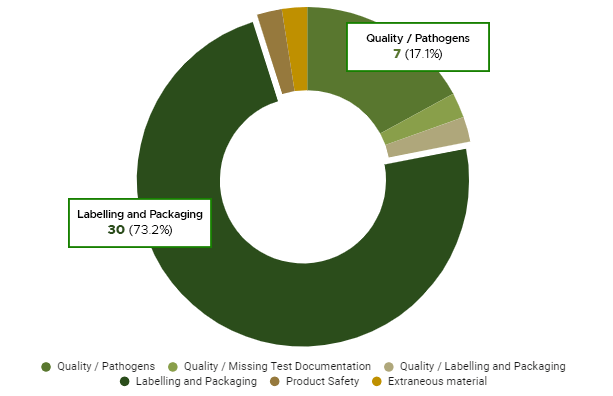
According to the data obtained in the last three years, the cannabis industry is still dealing with compliance issues. Looking at recall data over the last three years, we can see that labelling and packaging issues have caused 73 percent of recalls since October 2018. Pathogens account for 18% of all cannabis product recalls, making them the second most common cause. Despite being the second most frequently mentioned class, pathogens account for the greatest number of infected units [1].
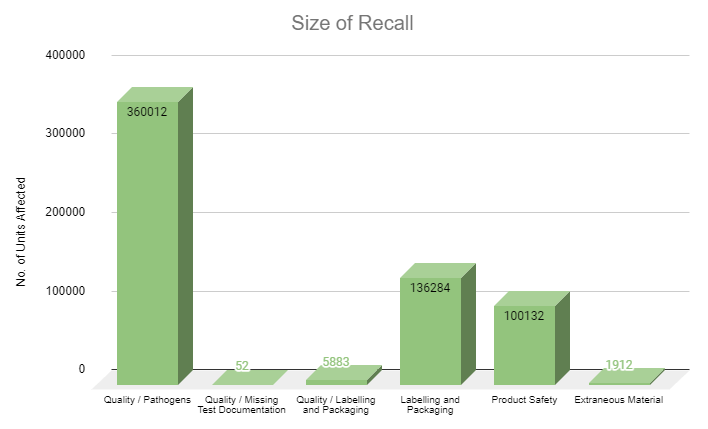
Canadian Cannabis Recalls – Total number of affected units and noted causes (Image Ref:https://cannabisindustryjournal.com/feature_article/cannabis- recalls-lessons-learned-after-three-years-of-Canadian-legalization/)
Labelling and packaging issues account for 73.2% of cannabis recalls since legalization in Canada (Image Ref:https://cannabisindustryjournal.com/ feature_article/cannabis-recalls-lessons-learned-after-three-years-of-Canadian- legalization/)
Recalls can be costly and lead to a loss of customer trust, but they are frequently avoidable. The challenge now is to figure out how to avoid recalls altogether.
It is more important than ever in today’s business environment to stay up to date on the rules and regulations that apply to your industry. Failure to do so can result in costly penalties such as product recalls and licence revocation.
Furthermore, staying up to date on the latest regulations can give you a competitive advantage over your competitors. It is critical to be up to date and informed about any changes in the regulations. Having up-to-date information on all regulatory changes will allow you to amend your documentation appropriately and in a timely manner, avoiding any potential recall.
Furthermore, in some cases, it is necessary to be aware of all applicable legislation. The Food and Drug Act and the Pest Control Products Regulations are two examples.

One important skill that is critical for success in many professions is the ability to read and interpret regulations. This can be a challenge, as regulations are often lengthy and filled with technical jargon. However, it is essential to be able to understand what the regulations say, as they can have a major impact on how you do your job.
Additionally, it is important to be able to interpret the regulations accurately, as even a small mistake can lead to significant consequences. You may not be able to enforce the regulations properly if you do not comprehend them.
Before drafting necessary documentation and executing the regulations, get the advice of an expert if you are having difficulty interpreting.

The first step in prevention is always to understand what you are attempting to prevent. For edibles and extracts, Health Canada requires the development of a preventative control plan (PCP). Identifying and mitigating all potential dangers, such as physical, biological, and chemical risks, is critical to the successful implementation of an effective PCP.
It is also critical that all batch documents are produced in such a way that all recognized hazards in the process are avoided throughout processing and that there is a verification step. This will undoubtedly reduce the number of pathogen-related recalls in edibles and extracts. Good Production Practices (GPP) and Good Agricultural Practices (GAP) can be implemented to avoid pathogen-related recalls in cannabis flowers and pre-rolls.
Cannabis GPP is a good practice guide that details how to appropriately cultivate, harvest, dry, and cure cannabis. Cannabis GPP also outlines how to store cannabis safely. One aspect of Cannabis GPP that is essential to preventing pathogen growth is ensuring that your formulation of cannabis products is not prone to pathogen growth.
Cannabis products can be either solid or liquid, and each type has different risks for pathogen contamination. For example, water can easily become contaminated with pathogens, so any wet cannabis products (such as oils) must be very carefully formulated to prevent the growth of pathogens. Similarly, dry cannabis products (such as flower) must also be formulated to prevent pathogen growth. Dry cannabis is less susceptible to contamination because it does not have the moisture that pathogens need to grow. As a result, it is essential to ensure that your formulation of cannabis products is not prone to pathogen growth in order to prevent the growth of pathogens. Shelf-life and/or stability studies are strongly suggested as part of product approval.
Any company that relies on raw materials or finished goods from suppliers needs to have a robust system in place for testing those products. While a Certificate of Analysis or Certificate of Conformance from a supplier can provide some assurance of quality, it’s always best to perform your own tests as well. That way, you can be sure that the product meets your specific requirements and that it’s safe for use.
There are many different ways to test products, but random testing is generally the most effective. You can ensure that the entire batch meets your standards by testing a small sample of products from each batch. The cost of testing is relatively low compared to the potential cost of using substandard materials, so it’s always worth the investment.
Furthermore, suppliers frequently employ a certificate of conformity (COC), which is just an administrative declaration that the product, according to the provider, meets the standard specification. Random testing of raw materials in-house can improve product quality and avert recalls.
It is critical to develop an SOP with a sampling plan that truly depicts the Batch. Furthermore, the execution of the written plan is critical. There is a risk if a plan is properly drafted but not executed.

By following these tips, you can help ensure that your batch records are accurate and compliant with GPPs.
It is also a good practice to create checklists for your batch documents to ensure compliance with all regulations. For example, to reduce the possibility of mislabeling, missing labelling information, and other issues, you can include several steps of label review and verification in your batch document.
GMP regulations mandate internal audits or self-inspections. On the other hand, the Canadian cannabis industry abides by GPP in compliance with the Cannabis Act and regulations, which do not need this. This can be incorporated into the quality system of authorized producers to reduce the likelihood of recalls. To avoid bias, hire a third-party expert to conduct self-inspections.

MFLRC is a one-stop shop for all of your quality assurance and compliance needs. Our team has years of experience in the cannabis industry and are expert in all facets. We offer a variety of services that will save you time and money. Let us take the burden off your shoulders so you can focus on what’s important – growing your business.
Some of our services include:
Contact us Now!
Email: info@mflrc.com
Call:1-647-544-7367
1. https://cannabisindustryjournal.com/feature_article/cannabis- recalls-lessons-learned-after-three-years-of-canadian-legalization/
Mussarat Fatima, President, and owner of MF Cannabis License and Regulatory Consultants has more than twenty years of experience in Quality Assurance, Quality Control, and Regulatory Affairs within the pharmaceutical, Food and Cannabis industries. She has a Master’s Degree in Food Sciences and Biochemistry; in addition to this, she also has a diploma in pharmaceutical Quality Assurance, Regulatory Affairs, and Quality Control. Also, she has completed several certifications specifically in Cannabis Quality Assurance, Regulatory Affairs, and Facility management from recognized institutes in Canada.

Written By: Mussarat Fatima
President at MF License & Regulatory Consultants
Website: https://mflrc.com/
Contact: info@mflrc.com